INTRODUCTION
Asparaginase-associated pancreatitis (AAP) is one of the most severe and dreaded complications during therapy of childhood acute lymphoblastic leukemia (ALL). It is characterized by intense abdominal pain, nausea/ vomiting, and systemic inflammatory response syndrome. AAP can result in chronic complications such as insulin dependence, exocrine pancreatic insufficiency, and chronic pain. These symptoms are more common when pancreatic necrosis or pancreatic pseudocysts develop immediately after AAP. Treatment for AAP has historically been limited to supportive care. However, a novel calcium release activated calcium channel blocker, zegocractin (formerly CM4620), currently in clinical trials in adults with pancreatitis, may reduce the severity of AAP. We thus developed the phase 1/2 CRSPA study to identify the recommended phase 2 dose (RP2D) of zegocractin in children and young adults with AAP during ALL treatment.
METHODS
To investigate the characteristics of AAP in the absence of zegocractin, we retrospectively evaluated patients treated on the Total Therapy XVI study (T16) who developed pancreatitis within 30 days of receiving asparaginase (AAP).
In the CRSPA trial, patients with ALL experiencing AAP were offered trial enrollment and provided informed consent/ assent. Participants underwent initial CT either prior to or on day 1 of CRSPA therapy. Zegocractin was administered as a 4-hour infusion on days 1-4 starting at dose level 1 (30mg/m 2 on day 1 and 42mg/m 2 on days 2-4), with dose limiting toxicities (DLT) defined as any CTCAE grade 3-5 toxicity not attributable to either pancreatitis or ALL therapy. Planned dose escalation was guided by a Keyboard design. The primary efficacy endpoint of the study focused on the occurrence of pancreatic necrosis or pseudocyst on CT after day 28.
RESULTS
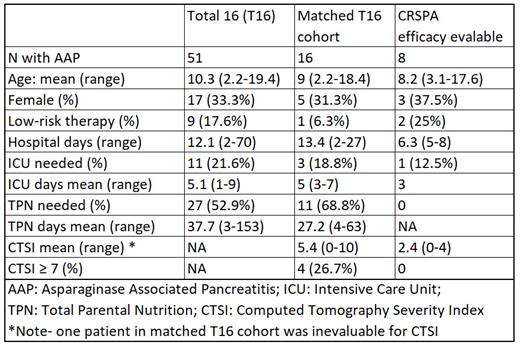
Among 598 patients treated on T16, 51 (8.5%) developed AAP. The mean age at AAP onset was 10.3 (range 2.2-21.4) years; 34 were male and 17 were female. 9 received low-risk therapy while the remainder were on standard- or high-risk therapy. Adequate follow-up imaging was available for 39 patients. Of these, 22 (56% of those with imaging, 43% of all AAP patients) had peri-pancreatic fluid collections (PPFC; N=18) and/or pancreatic necrosis (N=14). Mean hospitalization was 12.1 days (range 2-70), mean ICU time in the 11 (21.6%) patients requiring ICU care was 5.1 days (1-9), and mean duration of total parenteral nutrition (TPN) in the 27 (52.9%) patients needing it was 37.7 days (3-153).
Among the 9 patients (median age 8.8, range 3.1-17.6 years) treated in the first cohort of the Keyboard design phase 1 portion of CRSPA, 1 had a DLT (infusion reaction during dose 1) characterized by ill feeling and shortness of breath. The infusion was stopped and diphenhydramine was administered with symptomatic improvement. However, the symptoms recurred and worsened as the patient's AAP symptoms worsened. The event was retrospectively felt to be related to AAP rather than study medication, but it remained as a DLT. None of the other 8 patients treated at dose level 1 experienced a DLT. Pharmacokinetic analysis indicated that drug exposure at dose level 1 was at least as high as that seen in adults treated at the adult RP2D (i.e. 2 mg/kg on days 1-2 and 1.6 mg/kg on days 3-4). Because dose level 1 was well tolerated, we amended the protocol and evaluated whether there was a preliminary efficacy signal prior to dose escalation. Among the 8 patients who received all 4 doses and were evaluable for efficacy, 1 had a small PPFC and none had significant pancreatic necrosis on follow-up imaging; mean hospitalization was 6.3 days (range 5-8), 1 patient needed ICU care (3 days), and none required TPN.
To further assess preliminary efficacy, CTs from the T16 cohort were matched on sex and age with CRSPA patients at a 2:1 ratio and were evaluated by a blinded radiologist. The mean CT severity index was 5.4 in the historical controls vs. 2.4 in the CRSPA patients evaluable for efficacy.
CONCLUSION
Patients with AAP receiving supportive care in the historical T16 cohort experienced a high rate of pancreatic necrosis and pseudocyst. The CRSPA study established dose level 1 as the RP2D of zegocractin for children with ALL experiencing AAP. The phase 1 portion of the study has provided promising data on zegocractin efficacy. Moving forward, the ongoing phase 2 portion of the study will further assess and validate the efficacy of zegocractin in treating AAP.
OffLabel Disclosure:
Karol:Servier: Consultancy; Jazz Pharmaceuticals: Consultancy. Rubnitz:Biomea, Inc: Consultancy. Jeha:Amgen: Other: As part of the mission of St. Jude Global Hiroto Inaba, Victor Santana, Sima Jeha and Caitlyn Duffy participate in the Blincyto Humanitarian Access Program and provide in kind support for this program. . Relling:Servier: Consultancy. Inaba:Servier: Consultancy; Amgen: Other: As part of the mission of St. Jude Global Hiroto Inaba, Victor Santana, Sima Jeha and Caitlyn Duffy participate in the Blincyto Humanitarian Access Program and provide in kind support for this program..
Zegocractin is an investigational agent without an approved indication at this time.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal